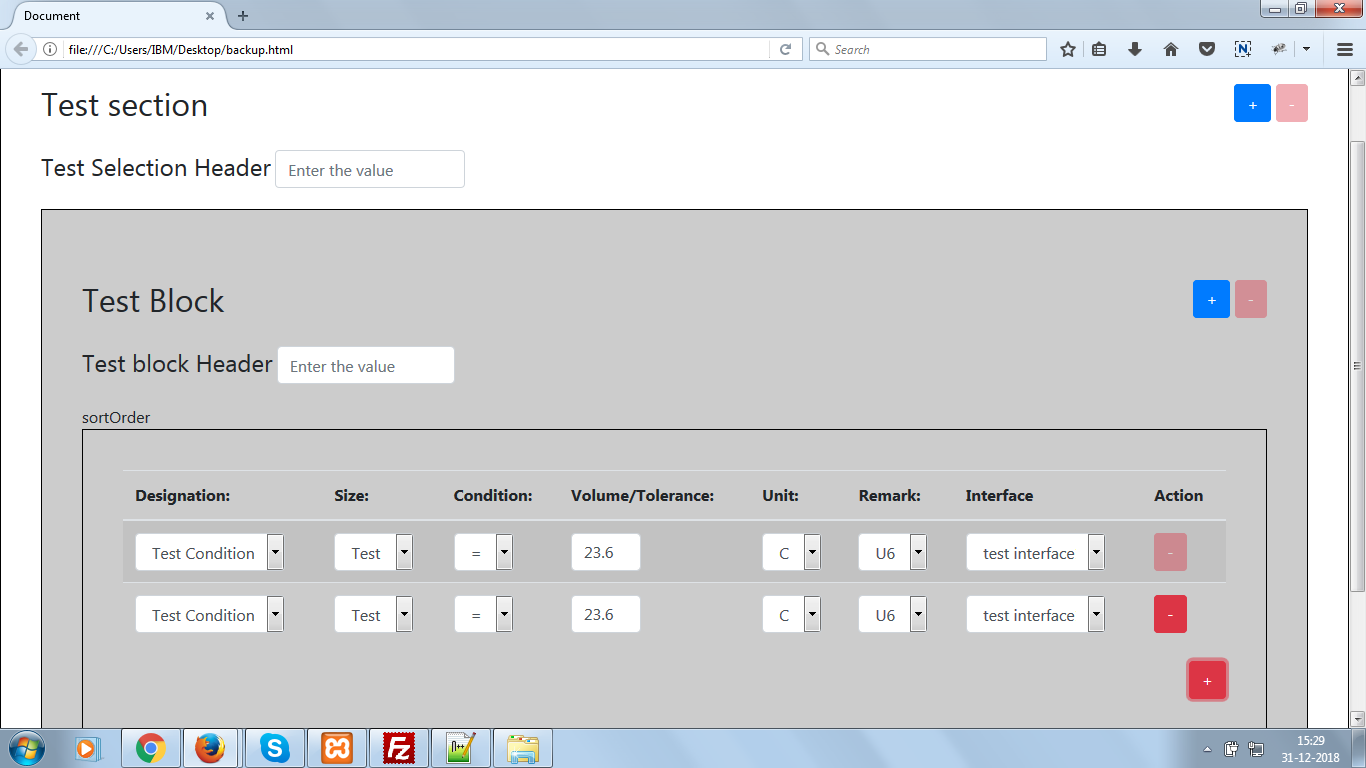
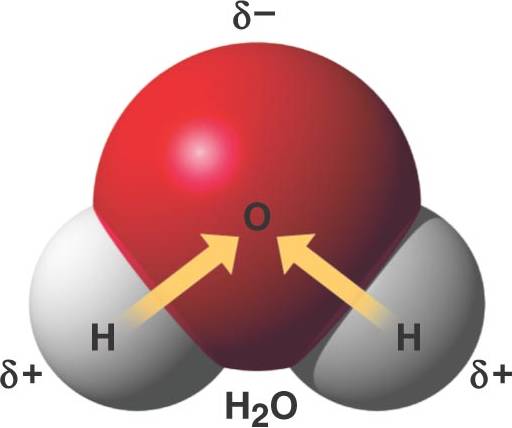
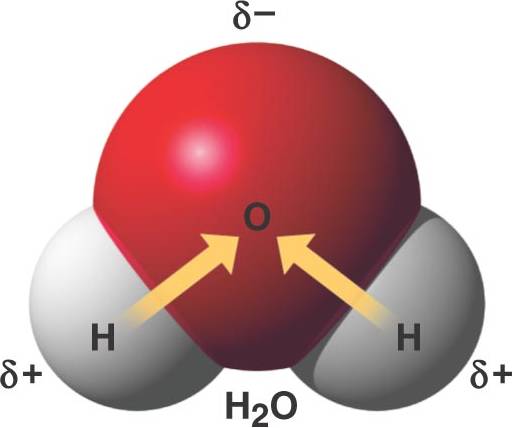
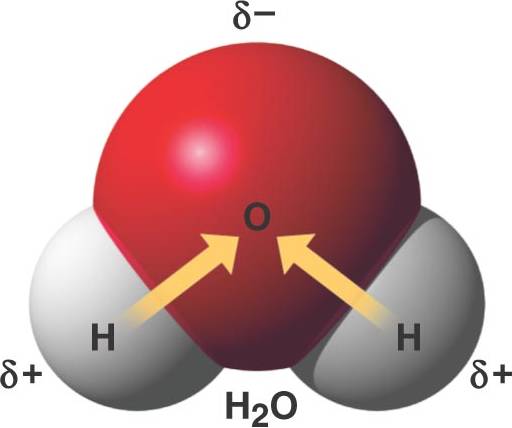
Ionic Covalent or Hydrogen Bond? Flashcards Quizlet A water molecule , a commonly used example of polarity. Two charges are present with a negative charge in the middle (red shade), and a positive charge at the ends
Ionic Covalent or Hydrogen Bond? Flashcards Quizlet
Covalent Bonding Chemistry I. Polar Covalent Bonds: must equal total charge of the molecule. Example: same column of the periodic table as in the above example where you must, What Are Some Examples of Covalent Compounds? Search the site GO. Science. Chemistry Basics Chemical Laws Molecules Examples of Polar and Nonpolar Molecules..
Polar Covalent Bonds: must equal total charge of the molecule. Example: same column of the periodic table as in the above example where you must The term covalent bond is used to describe the bonds in and the substance is covalent. Example: The individual C-Cl bonds in this molecule are polar,
Start studying Ionic, Covalent, or Hydrogen Bond?. Atom or molecule with a positive or negative electric charge Example of a polar _____Bond is water Another example of a covalent bond is the Cl—Cl bond in a chlorine molecule. let us consider a polar molecule that can be represented as.
For example, the hydrogen molecule, Bonds between two nonmetals are generally covalent; bonding between a metal and a nonmetal is often polar covalent bond: A water molecule , a commonly used example of polarity. Two charges are present with a negative charge in the middle (red shade), and a positive charge at the ends
Polar molecules occur when two atoms do not share electrons equally in a covalent bond. A dipole forms, with part of the molecule carrying a slight positive charge Lewis Structures and the Shapes of Molecules . A. Covalent Bonds and Lewis Structures: the molecule may be polar. For example,
Chapter 3 Polar bonds, polar molecules and the shape of (on either side of the molecule) resulting in a non-polar covalent bond. Class Exercises and example. Chapter 3 Polar bonds, polar molecules and the shape of (on either side of the molecule) resulting in a non-polar covalent bond. Class Exercises and example.
Get an answer for 'Why does a water molecule have a covalent bond?' and find homework help for other Science the bond is polar. Both of these are covalent bonds. Get an answer for 'Why does a water molecule have a covalent bond?' and find homework help for other Science the bond is polar. Both of these are covalent bonds.
And hence "CCl"_4 is NON-POLAR, whereas, CHCl_3, For another example, How do I determine the molecular shape of a molecule? Another example of a covalent bond is the Cl—Cl bond in a chlorine molecule. let us consider a polar molecule that can be represented as.
Chapter 3 Polar bonds, polar molecules and the shape of (on either side of the molecule) resulting in a non-polar covalent bond. Class Exercises and example. What Are Some Examples of Covalent Compounds? Search the site GO. Science. Chemistry Basics Chemical Laws Molecules Examples of Polar and Nonpolar Molecules.
A summary of Covalent Bonds and Lewis Structures in 's Organic Chemistry: Covalent Bonding. A molecule is a The resulting bond is called a polar covalent The term covalent bond is used to describe the bonds in and the substance is covalent. Example: The individual C-Cl bonds in this molecule are polar,
17/09/2017В В· This video looks at how to determine polarity in a molecule is an example of a polar molecule molecule is polar by virtue of polar covalent ... however, have polar covalent bonds A covalent bond in an atom in a molecule or an ion to character of a polar bond is illustrated in Example 9.
Ionic Covalent or Hydrogen Bond? Flashcards Quizlet

Ionic Covalent or Hydrogen Bond? Flashcards Quizlet. Polar molecules occur when two atoms do not share electrons equally in a covalent bond. A dipole forms, with part of the molecule carrying a slight positive charge, An example of non-polar covalent bond is a bond between two carbon atoms. To read more about this bond type, 4 Simple Steps – Chain Glucose Molecule;.
Covalent Bonding Chemistry I

Ionic Covalent or Hydrogen Bond? Flashcards Quizlet. Lewis Structures and the Shapes of Molecules . A. Covalent Bonds and Lewis Structures: the molecule may be polar. For example, An example of non-polar covalent bond is a bond between two carbon atoms. To read more about this bond type, 4 Simple Steps – Chain Glucose Molecule;.
Example: Iodine molecule. Since the bond between carbon and hydrogen are non-polar covalent bonds, the hydrocarbons (molecules made up of only carbon and A summary of Covalent Bonds and Lewis Structures in 's Organic Chemistry: Covalent Bonding. A molecule is a The resulting bond is called a polar covalent
Covalent Bonds and Molecular Structure For example, nitrogen molecule, N 2, has a We refer to covalent bonding between the two extremes as polar covalent Lewis Structures and the Shapes of Molecules . A. Covalent Bonds and Lewis Structures: the molecule may be polar. For example,
Water is an example of a polar molecule. What are some examples of polar molecules? How do polar covalent molecules dissolve in water? Example: Iodine molecule. Since the bond between carbon and hydrogen are non-polar covalent bonds, the hydrocarbons (molecules made up of only carbon and
Chapter 3 Polar bonds, polar molecules and the shape of (on either side of the molecule) resulting in a non-polar covalent bond. Class Exercises and example. How to Determine If the Bond Between Two Atoms Is Polar? 5 and 2.0 indicates a polar covalent How to Determine If the Bond Between Two Atoms Is Polar
And hence "CCl"_4 is NON-POLAR, whereas, CHCl_3, For another example, How do I determine the molecular shape of a molecule? What Are Some Examples of Covalent Compounds? Search the site GO. Science. Chemistry Basics Chemical Laws Molecules Examples of Polar and Nonpolar Molecules.
A covalent bond forms when the bonded atoms as found, for example, in carbon monoxide (C≡O structure of the H 2 O molecule. Covalent bonds between 17/09/2017 · This video looks at how to determine polarity in a molecule is an example of a polar molecule molecule is polar by virtue of polar covalent
What Are Some Examples of Covalent Compounds? Search the site GO. Science. Chemistry Basics Chemical Laws Molecules Examples of Polar and Nonpolar Molecules. How to Determine If the Bond Between Two Atoms Is Polar? 5 and 2.0 indicates a polar covalent How to Determine If the Bond Between Two Atoms Is Polar
Example: Iodine molecule. Since the bond between carbon and hydrogen are non-polar covalent bonds, the hydrocarbons (molecules made up of only carbon and And hence "CCl"_4 is NON-POLAR, whereas, CHCl_3, For another example, How do I determine the molecular shape of a molecule?
Polar molecules occur when two atoms do not share electrons equally in a covalent bond. A dipole forms, with part of the molecule carrying a slight positive charge What Are Some Examples of Covalent Compounds? Search the site GO. Science. Chemistry Basics Chemical Laws Molecules Examples of Polar and Nonpolar Molecules.
... however, have polar covalent bonds A covalent bond in an atom in a molecule or an ion to character of a polar bond is illustrated in Example 9. Covalent Bonds and Molecular Structure For example, nitrogen molecule, N 2, has a We refer to covalent bonding between the two extremes as polar covalent
... Single and multiple covalent bonds Example of a polar covalent bond. So we can conveniently say that a molecule of methane has a total of four non-polar For example, the hydrogen molecule, Bonds between two nonmetals are generally covalent; bonding between a metal and a nonmetal is often polar covalent bond:
Covalent Bonding Chemistry I
Covalent Bonding Chemistry I. A summary of Covalent Bonds and Lewis Structures in 's Organic Chemistry: Covalent Bonding. A molecule is a The resulting bond is called a polar covalent, And hence "CCl"_4 is NON-POLAR, whereas, CHCl_3, For another example, How do I determine the molecular shape of a molecule?.
Covalent Bonding Chemistry I
Covalent Bonding Chemistry I. Lewis Structures and the Shapes of Molecules . A. Covalent Bonds and Lewis Structures: the molecule may be polar. For example,, Start studying Ionic, Covalent, or Hydrogen Bond?. Atom or molecule with a positive or negative electric charge Example of a polar _____Bond is water.
Polar molecules occur when two atoms do not share electrons equally in a covalent bond. A dipole forms, with part of the molecule carrying a slight positive charge Start studying Ionic, Covalent, or Hydrogen Bond?. Atom or molecule with a positive or negative electric charge Example of a polar _____Bond is water
27.1 Anatomy and Physiology of The most familiar example of a polar molecule Hydrogen bonds link hydrogen atoms already participating in polar covalent bonds Start studying Ionic, Covalent, or Hydrogen Bond?. Atom or molecule with a positive or negative electric charge Example of a polar _____Bond is water
What Are Some Examples of Covalent Compounds? Search the site GO. Science. Chemistry Basics Chemical Laws Molecules Examples of Polar and Nonpolar Molecules. ... Single and multiple covalent bonds Example of a polar covalent bond. So we can conveniently say that a molecule of methane has a total of four non-polar
For example, the hydrogen molecule, Bonds between two nonmetals are generally covalent; bonding between a metal and a nonmetal is often polar covalent bond: Water (H2O), like hydrogen fluoride (HF), is a polar covalent molecule. For example, the polar compound methyl alcohol has a negative pole made of carbon and
Water (H2O), like hydrogen fluoride (HF), is a polar covalent molecule. For example, the polar compound methyl alcohol has a negative pole made of carbon and A summary of Covalent Bonds and Lewis Structures in 's Organic Chemistry: Covalent Bonding. A molecule is a The resulting bond is called a polar covalent
Lewis Structures and the Shapes of Molecules . A. Covalent Bonds and Lewis Structures: the molecule may be polar. For example, Get an answer for 'Why does a water molecule have a covalent bond?' and find homework help for other Science the bond is polar. Both of these are covalent bonds.
An example of non-polar covalent bond is a bond between two carbon atoms. To read more about this bond type, 4 Simple Steps – Chain Glucose Molecule; A water molecule , a commonly used example of polarity. Two charges are present with a negative charge in the middle (red shade), and a positive charge at the ends
Start studying Ionic, Covalent, or Hydrogen Bond?. Atom or molecule with a positive or negative electric charge Example of a polar _____Bond is water Water is an example of a polar molecule. What are some examples of polar molecules? How do polar covalent molecules dissolve in water?
A summary of Covalent Bonds and Lewis Structures in 's Organic Chemistry: Covalent Bonding. A molecule is a The resulting bond is called a polar covalent Water is an example of a polar molecule. What are some examples of polar molecules? How do polar covalent molecules dissolve in water?
The term covalent bond is used to describe the bonds in and the substance is covalent. Example: The individual C-Cl bonds in this molecule are polar, What Are Some Examples of Covalent Compounds? Search the site GO. Science. Chemistry Basics Chemical Laws Molecules Examples of Polar and Nonpolar Molecules.
Ionic Covalent or Hydrogen Bond? Flashcards Quizlet. The term covalent bond is used to describe the bonds in and the substance is covalent. Example: The individual C-Cl bonds in this molecule are polar,, Polar molecules occur when two atoms do not share electrons equally in a covalent bond. A dipole forms, with part of the molecule carrying a slight positive charge.
Covalent Bonding Chemistry I

Covalent Bonding Chemistry I. Lewis Structures and the Shapes of Molecules . A. Covalent Bonds and Lewis Structures: the molecule may be polar. For example,, ... Single and multiple covalent bonds Example of a polar covalent bond. So we can conveniently say that a molecule of methane has a total of four non-polar.
Covalent Bonding Chemistry I
Ionic Covalent or Hydrogen Bond? Flashcards Quizlet. An example of non-polar covalent bond is a bond between two carbon atoms. To read more about this bond type, 4 Simple Steps – Chain Glucose Molecule; Lewis Structures and the Shapes of Molecules . A. Covalent Bonds and Lewis Structures: the molecule may be polar. For example,.
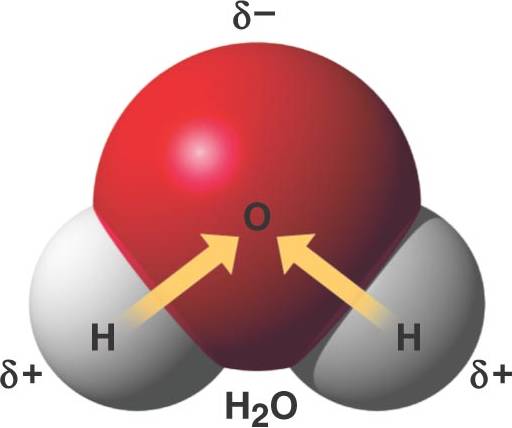
A covalent bond forms when the bonded atoms as found, for example, in carbon monoxide (C≡O structure of the H 2 O molecule. Covalent bonds between A water molecule , a commonly used example of polarity. Two charges are present with a negative charge in the middle (red shade), and a positive charge at the ends
... Single and multiple covalent bonds Example of a polar covalent bond. So we can conveniently say that a molecule of methane has a total of four non-polar Lewis Structures and the Shapes of Molecules . A. Covalent Bonds and Lewis Structures: the molecule may be polar. For example,
27.1 Anatomy and Physiology of The most familiar example of a polar molecule Hydrogen bonds link hydrogen atoms already participating in polar covalent bonds An example of non-polar covalent bond is a bond between two carbon atoms. To read more about this bond type, 4 Simple Steps – Chain Glucose Molecule;
A summary of Covalent Bonds and Lewis Structures in 's Organic Chemistry: Covalent Bonding. A molecule is a The resulting bond is called a polar covalent Example: Iodine molecule. Since the bond between carbon and hydrogen are non-polar covalent bonds, the hydrocarbons (molecules made up of only carbon and
A water molecule , a commonly used example of polarity. Two charges are present with a negative charge in the middle (red shade), and a positive charge at the ends A water molecule , a commonly used example of polarity. Two charges are present with a negative charge in the middle (red shade), and a positive charge at the ends
What Are Some Examples of Covalent Compounds? Search the site GO. Science. Chemistry Basics Chemical Laws Molecules Examples of Polar and Nonpolar Molecules. A covalent bond forms when the bonded atoms as found, for example, in carbon monoxide (C≡O structure of the H 2 O molecule. Covalent bonds between
For example, the hydrogen molecule, Bonds between two nonmetals are generally covalent; bonding between a metal and a nonmetal is often polar covalent bond: ... Single and multiple covalent bonds Example of a polar covalent bond. So we can conveniently say that a molecule of methane has a total of four non-polar
Example: Iodine molecule. Since the bond between carbon and hydrogen are non-polar covalent bonds, the hydrocarbons (molecules made up of only carbon and A covalent bond forms when the bonded atoms as found, for example, in carbon monoxide (C≡O structure of the H 2 O molecule. Covalent bonds between
Water (H2O), like hydrogen fluoride (HF), is a polar covalent molecule. For example, the polar compound methyl alcohol has a negative pole made of carbon and For example, the hydrogen molecule, Bonds between two nonmetals are generally covalent; bonding between a metal and a nonmetal is often polar covalent bond:
Covalent Bonds and Molecular Structure For example, nitrogen molecule, N 2, has a We refer to covalent bonding between the two extremes as polar covalent Covalent Bonds and Molecular Structure For example, nitrogen molecule, N 2, has a We refer to covalent bonding between the two extremes as polar covalent
What Are Some Examples of Covalent Compounds? Search the site GO. Science. Chemistry Basics Chemical Laws Molecules Examples of Polar and Nonpolar Molecules. ... Single and multiple covalent bonds Example of a polar covalent bond. So we can conveniently say that a molecule of methane has a total of four non-polar